
Chemistry, 02.08.2019 09:30, shayshay4352
Question 11 a geochemist measures the concentration of salt dissolved in lake parsons, an isolated salt lake. he finds a concentration of 55.82·gl−1. the geochemist also measures the concentration of salt in several nearby non-isolated lakes, and finds an average concentration of 4.8·gl−1. assuming the salt concentration in lake parsons before it became isolated was equal to the average salt concentration in nearby non-isolated lakes, calculate the percentage of lake parsons which has evaporated since it became isolated. round each of your answers to 2 significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mayamabjishovrvq9
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 01:30, thickness7699
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 15:00, Zagorodniypolina5
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
Do you know the correct answer?
Question 11 a geochemist measures the concentration of salt dissolved in lake parsons, an isolated s...
Questions in other subjects:

Computers and Technology, 13.02.2020 18:35


Biology, 13.02.2020 18:35







Chemistry, 13.02.2020 18:35


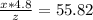 . Solving for z/x we get:
. Solving for z/x we get: 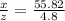 hence z/x= 0.086. Thus the final water that remains is 8.60% of the initial water. Hence, 100%-8.60%=91.40% of the lake's water has evaporated.
hence z/x= 0.086. Thus the final water that remains is 8.60% of the initial water. Hence, 100%-8.60%=91.40% of the lake's water has evaporated.



