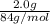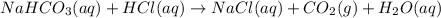If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon dioxide should one tablet yield? compare this theoretical value with your results. (hint: you will first need to convert your mass into moles by dividing by the molar mass of nahco3 or sodium hydrogen carbonate a/k/a baking soda). , show all work and think about mole ratios relating co2 to nahco3.

Answers: 1
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 07:30, lucas2020197
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
Do you know the correct answer?
If a typical antacid tablet contains 2.0 g of sodium hydrogen carbonate, how many moles of carbon di...
Questions in other subjects:



English, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20


English, 22.04.2021 21:20




English, 22.04.2021 21:20


 (1)
(1)