Given the reaction:
ch4(g) + 2 o2(g) --> 2 h2o(g) + co2(g)
what is the overall resul...

Chemistry, 06.10.2019 01:30, hayleylatti8691
Given the reaction:
ch4(g) + 2 o2(g) --> 2 h2o(g) + co2(g)
what is the overall result when ch4(g) burns
according to this reaction?
(1) energy is absorbed and dh is negative.
(2) energy is absorbed and dh is positive.
(3) energy is released and dh is negative.
(4) energy is released and dh is positive.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1


Do you know the correct answer?
Questions in other subjects:

Mathematics, 13.10.2019 22:30

Mathematics, 13.10.2019 22:30

English, 13.10.2019 22:30

Mathematics, 13.10.2019 22:30





English, 13.10.2019 22:30

Mathematics, 13.10.2019 22:30


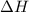 is negative.
is negative.



