
Chemistry, 02.08.2019 22:30, rubimachuca1020
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.35 m, and the concentration of h2so3 is 0.23 m. this reaction a. is in equilibrium b. must shift to the reactants to be in equilibrium c. must shift to the products to be in equilibrium d. must have the pressure increased to reach equilibrium e. none of the above

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3
Do you know the correct answer?
In the reaction so2 (g) + h2o (l) ↔ h2so3 (aq), with k = 2.1 × 10–3, the concentration of so2 is 0.3...
Questions in other subjects:

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Physics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Social Studies, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01

English, 16.09.2020 05:01

Geography, 16.09.2020 05:01

Mathematics, 16.09.2020 05:01


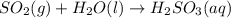
![Q=\frac{[H_2SO_3(aq)]}{[SO_2(g)]}](/tpl/images/0163/3606/a76c3.png)
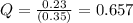
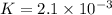
 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.
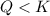 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.
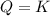 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.



