
Chemistry, 03.08.2019 05:30, krystlemiller4307
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 . if the molar mass of the compound is 129.1 g/mol, what is the chemical formula of the compound?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 23.06.2019 03:40, ElegantEmerald
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
Do you know the correct answer?
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 ....
Questions in other subjects:



Mathematics, 12.01.2021 15:10





Mathematics, 12.01.2021 15:10



 . The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
. The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.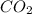 , 1.09 g of
, 1.09 g of 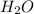 and 1.70 g of
and 1.70 g of 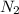 . First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
. First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.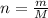
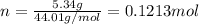
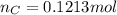
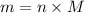
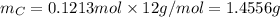
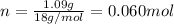

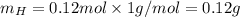
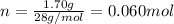





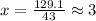

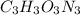 .
.



