
Me i beg you
the average kinetic energy of 1 mole of a gas at -32 degrees celsius is:
3.01 x 103 j
3.99 x 102 j
-3.99 x 102 j
3.80 x 103 j
the relationship between volume and temperature of a gas, when pressure and moles of a gas are held constant, is: v*t = k.
true
false
the relationship between moles and volume, when pressure and temperature of a gas are held constant, is: v/n = k. we could say then, that:
if the moles of gas are tripled, the volume must also triple.
if the moles of gas are halved, the volume must be doubled.
if the moles of gas are doubled, the volume must be halved.
if the moles of gas are doubled, the volume is unaffected.
if the temperature and volume of a gas are held constant, an increase in pressure would most likely be caused by an increase in the number of moles of gas.
true
false
if the vapor pressure of a liquid is less than the atmospheric pressure, the liquid will not boil.
true
false
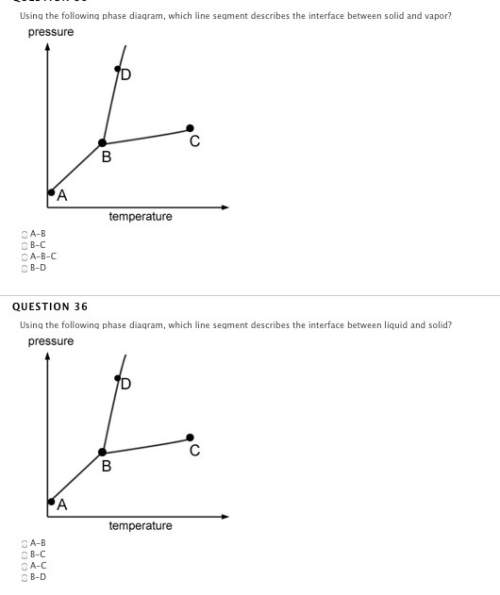

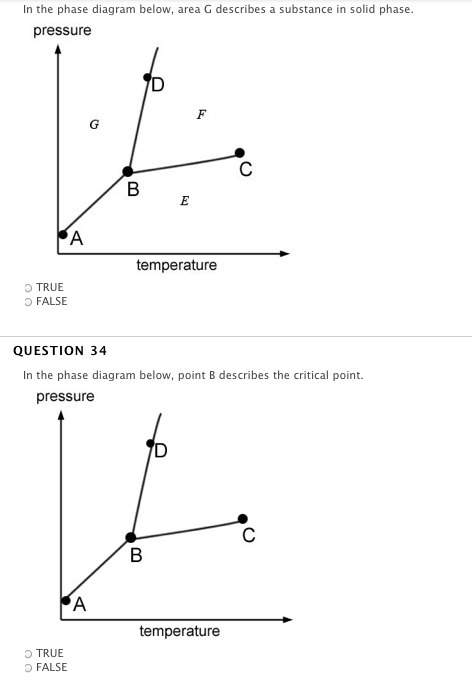
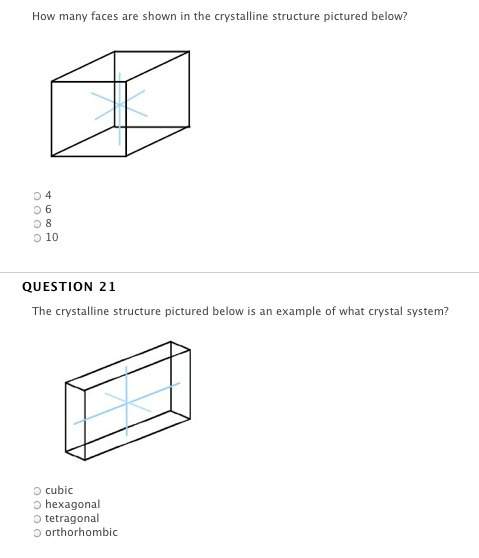

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, jamiesong3501
What is the partial pressure of ethane, pethane, in the flask?
Answers: 1



Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1
Do you know the correct answer?
Me i beg you
the average kinetic energy of 1 mole of a gas at -32 degrees celsius is: <...
the average kinetic energy of 1 mole of a gas at -32 degrees celsius is: <...
Questions in other subjects:


Chemistry, 20.11.2020 20:10





Mathematics, 20.11.2020 20:10

Mathematics, 20.11.2020 20:10


Health, 20.11.2020 20:10






