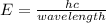Chemistry, 03.08.2019 17:30, rachelreed
When an electron of an atom falls from a higher energy level to the ground state, the atom loses 9.4145 x 10-25 joules of energy. what is the wavelength of the radiation emitted as a result of this transition? (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108m/s)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1

Chemistry, 23.06.2019 09:30, oscarruiz
The allotropes of carbon include a variety of structures that include three-dimensional tetrahedral lattices, planes of hexagonal rings, cylindrical tubes of hexagonal rings, and spheres of five- and six-membered rings. similar shapes of network covalent atomic solids are possible with carbon nitride, boron, and pure silicon (e. g., silicene is a graphene-like allotrope of pure silicon). in contrast, silicates exist as either highly ordered or amorphous (more random) three-dimensional lattices. what could explain why there are there no naturally occurring sheets, stacked sheets, cylindrical tubes, or spheres of network covalent atomic solids composed of silicon and oxygen (sio2)? would pure silicate structures make good lubricants or good electrical conductors?
Answers: 3

Chemistry, 23.06.2019 14:00, 1940swannabe
Which word refers to the smallest functional unit of living thing
Answers: 1
Do you know the correct answer?
When an electron of an atom falls from a higher energy level to the ground state, the atom loses 9.4...
Questions in other subjects:


Chemistry, 01.10.2019 02:00

History, 01.10.2019 02:00


History, 01.10.2019 02:00


Chemistry, 01.10.2019 02:00



Biology, 01.10.2019 02:00