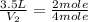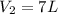The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(g). if a balloon was filled with 3.5l of ammonia gas and all of the gas decomposed, what would be the total volume of gas in the container? assume the pressure and temp remains constant.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, earcake2470
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
Do you know the correct answer?
The decomposition of ammonia gas (a process that occurs at high temps) is 2kh3(g) --> n2(g) 3h2(...
Questions in other subjects:

Mathematics, 03.02.2020 14:02



English, 03.02.2020 14:02

Mathematics, 03.02.2020 14:02






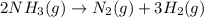
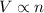
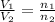
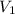 = initial volume of ammonia gas = 3.5 L
= initial volume of ammonia gas = 3.5 L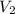 = total volume of gas in the container = ?
= total volume of gas in the container = ?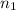 = initial moles of ammonia gas = 2 mole
= initial moles of ammonia gas = 2 mole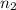 = final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles
= final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles