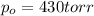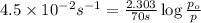Chemistry, 04.08.2019 08:00, dbn4everloved8
The decomposition of sulfuryl chloride (so2cl2) is a first-order process. the rate constant for the decomposition at 660 k is 4.5 ✕ 10−2 s-1. (a) if we begin with an initial so2cl2 pressure of 430. torr, what is the pressure of this substance after 70. s?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1


Do you know the correct answer?
The decomposition of sulfuryl chloride (so2cl2) is a first-order process. the rate constant for the...
Questions in other subjects:





Mathematics, 21.01.2021 21:20




Mathematics, 21.01.2021 21:20


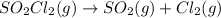
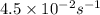
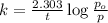
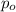 = Initial pressure of the gas
= Initial pressure of the gas