
Chemistry, 04.08.2019 10:30, ambarpena14
Agas is sealed in a bottle at 25°c and 1.50 atm pressure. how must the storage conditions be changed to bring the gas to standard temperature and pressure (stp)? increase the temperature by 25°c and decrease the pressure by 0.514 atm. keep the temperature the same and increase the pressure by 98.5 atm. decrease the temperature by 25°c and decrease the pressure by 0.500 atm.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, kimberlyrios12p0ts98
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Do you know the correct answer?
Agas is sealed in a bottle at 25°c and 1.50 atm pressure. how must the storage conditions be changed...
Questions in other subjects:

Biology, 20.04.2020 21:02

History, 20.04.2020 21:02



History, 20.04.2020 21:02

Social Studies, 20.04.2020 21:02





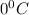 and an absolute pressure of exactly 1 atm.
and an absolute pressure of exactly 1 atm.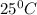 and 1.50 atmosphere to bring to STP , the temperature must be decreased by
and 1.50 atmosphere to bring to STP , the temperature must be decreased by 



