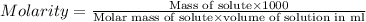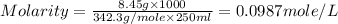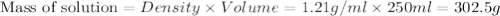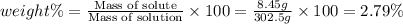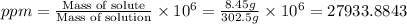Chemistry, 04.08.2019 17:30, fredvales19
Asolution of a sugar with the chemical formula (c12h22o11) is prepared by dissolving 8.45 g in 250.0 ml of water at 25 c. the density of this solution is 1.21 g/cm3. calculate the concentration in terms of molarity, molality, weight percent and ppm. assume that the volume of the solution is equal to the volume of the solvent. show your work.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
Do you know the correct answer?
Asolution of a sugar with the chemical formula (c12h22o11) is prepared by dissolving 8.45 g in 250.0...
Questions in other subjects:

Mathematics, 10.07.2019 00:30


Mathematics, 10.07.2019 00:30


History, 10.07.2019 00:30




Mathematics, 10.07.2019 00:30


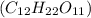 = 8.45 g
= 8.45 g