
Chemistry, 04.08.2019 22:30, kyliemorgan8623
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg ab (fast) and the rate = k[a][b] step 2: ab + b mc016-2.jpg ab2 (slow) and the rate = k[ab][b] step 3: ab2 + b mc016-3.jpg ab3 (fast) and the rate = k[ab2][b] overall: a + 3b mc016-4.jpg ab3 and the rate = k[a][b]2 which explains why the rate law for the overall equation is not the same as the rate equation for the rate-determining step?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 22.06.2019 07:30, tntaylor862
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1
Do you know the correct answer?
Consider the following three-step representation of a reaction mechanism. step 1: a + b mc016-1.jpg...
Questions in other subjects:


Mathematics, 16.07.2020 01:01


Mathematics, 16.07.2020 01:01

Computers and Technology, 16.07.2020 01:01



Mathematics, 16.07.2020 01:01

Mathematics, 16.07.2020 01:01

Arts, 16.07.2020 01:01


![A+B\rightleftharpoons AB(fast)\\Rate=K[A][B]](/tpl/images/0171/0396/8b588.png)
![AB+B\rightarrow AB_2(slow)\\Rate=K[AB][B]](/tpl/images/0171/0396/fb2ef.png)
![AB_2+B\rightleftharpoons AB_3(fast)\\Rate=K[AB_2][B]](/tpl/images/0171/0396/ac6cb.png)
![Rate=K[A][B]^2](/tpl/images/0171/0396/92d0b.png)
![Rate=K[AB][B]](/tpl/images/0171/0396/058ce.png) ...........(A)
...........(A)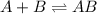
![K=\frac{[AB]}{[A][B]}](/tpl/images/0171/0396/6ec78.png)
![[AB]=K[A][B]](/tpl/images/0171/0396/ad806.png)
![Rate=KK[A][B][B]](/tpl/images/0171/0396/3919b.png)
![Rate=K'[A][B]^2](/tpl/images/0171/0396/8db87.png)




