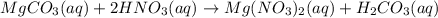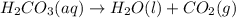Chemistry, 04.08.2019 16:30, aharvitt0417
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid form. carbonic acid then breaks down into water and carbon dioxide. what two types of reactions take place in this process?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
Do you know the correct answer?
When magnesium carbonate, mgco2, reacts with nitric acid, hno3, magnesium nitrate and carbonic acid...
Questions in other subjects:

English, 07.02.2021 01:50


Mathematics, 07.02.2021 01:50



Mathematics, 07.02.2021 01:50

Mathematics, 07.02.2021 01:50


Social Studies, 07.02.2021 01:50

Mathematics, 07.02.2021 01:50