
The electronic configuration of an element is given below. 1s22s22p63s1 which statement about the reactivity of the element is true? it is reactive because it has a full outermost energy level. it is unreactive because it has a full outermost energy level. it is reactive because it has to lose one electron to have a full outermost energy level. it is unreactive because it has to gain one electron to have a full outermost energy level.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 16:00, esme06quirino
Is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 2

Do you know the correct answer?
The electronic configuration of an element is given below. 1s22s22p63s1 which statement about the re...
Questions in other subjects:



Mathematics, 05.05.2020 13:56



Mathematics, 05.05.2020 13:56



Mathematics, 05.05.2020 13:56


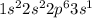 shows that there is only one valence electron present in the outermost shell.
shows that there is only one valence electron present in the outermost shell.



