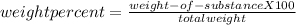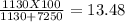Making homemade ice cream is one of life's great pleasures. fresh milk and cream, sugar, and flavorings are churned in a bucket suspended in an ice–water mixture, the freezing point of which has been lowered by adding rock salt. one manufacturer of home ice cream freezers recommends adding 2.50 lb (1130 g) of rock salt (nacl) to 16.0 lb of ice (7250 g) in a 4-qt freezer. calculate the weight percent of nacl of the solution that will result when this mixture melts.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 23.06.2019 05:30, victoria6929
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
Do you know the correct answer?
Making homemade ice cream is one of life's great pleasures. fresh milk and cream, sugar, and flavori...
Questions in other subjects:



History, 27.04.2020 02:22

English, 27.04.2020 02:22

Computers and Technology, 27.04.2020 02:22

Mathematics, 27.04.2020 02:22


Mathematics, 27.04.2020 02:22

Mathematics, 27.04.2020 02:22