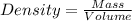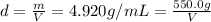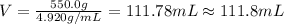Chemistry, 04.08.2019 00:00, andershy1405
Asample of an unknown metal (density = 4.920 g/ml) weighs 550.0 g. what is the volume of this piece of metal? a. 111.8 ml b. none of these c. 2.706 × 10^3 ml d. 1.118 × 10^5 ml e. 8.945 × 10^–3 ml

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:10, hadellolo8839
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
Do you know the correct answer?
Asample of an unknown metal (density = 4.920 g/ml) weighs 550.0 g. what is the volume of this piece...
Questions in other subjects:









Mathematics, 29.07.2019 17:00