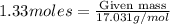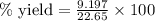Chemistry, 01.08.2019 21:30, 05leslun42715
Ammonia (nh3) can be produced by the reaction of hydrogen gas with nitrogen gas: 3h2 + n2 = 2nh3 a chemist reacts 2.00 mol h2 with excess n2. the reaction yields 0.54 mol nh3. what is the percent yield of the reaction? a) 25% b) 40% c) 60% d) 80%

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, melidacampos12
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 14:40, neonbluefaith
Which statement best describes the function of enzymes?
Answers: 1
Do you know the correct answer?
Ammonia (nh3) can be produced by the reaction of hydrogen gas with nitrogen gas: 3h2 + n2 = 2nh3 a...
Questions in other subjects:











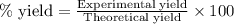 ....(1)
....(1)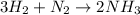
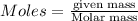 ....(2)
....(2)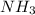 = 17.031g/mol
= 17.031g/mol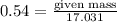
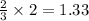 moles of ammonia.
moles of ammonia.