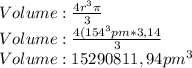Chemistry, 01.08.2019 05:00, Tyrant4life
Given the atomic radius of neon, 0.69 å, and knowing that a sphere has a volume of 4πr3/3, calculate the fraction of space that ne atoms occupy in a sample of neon at stp.

Answers: 1
Similar questions


Chemistry, 10.07.2019 10:50, stankyweezle
Answers: 2

Mathematics, 12.07.2019 03:00, mdegracia73
Answers: 1

Chemistry, 13.08.2019 05:10, gachaperson123
Answers: 2
Do you know the correct answer?
Given the atomic radius of neon, 0.69 å, and knowing that a sphere has a volume of 4πr3/3, calculate...
Questions in other subjects:

Physics, 11.05.2020 06:57

Mathematics, 11.05.2020 07:57


Mathematics, 11.05.2020 07:57



Mathematics, 11.05.2020 07:57

Physics, 11.05.2020 07:57

Mathematics, 11.05.2020 07:57