
Chemistry, 29.07.2019 14:30, JAXKBOII213
Which of the following circumstances will result in a reaction that is spontaneous only at high temperatures? a. positive enthalpy change and positive entropy change b. negative enthalpy change and negative entropy change c. positive enthalpy change and negative entropy change d. negative enthalpy change and positive entropy change

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, coreyslotte
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2


Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
Do you know the correct answer?
Which of the following circumstances will result in a reaction that is spontaneous only at high temp...
Questions in other subjects:

History, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30


Mathematics, 12.02.2021 20:30



Geography, 12.02.2021 20:30

History, 12.02.2021 20:30


Mathematics, 12.02.2021 20:30


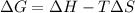
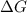 = Gibbs free energy
= Gibbs free energy 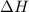 = enthalpy change
= enthalpy change
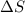 = entropy change
= entropy change 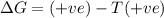
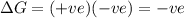 only when
only when  is higher which is possible at high temperatures
is higher which is possible at high temperatures



