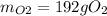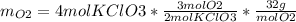The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info: mm o2 = 32 g/mol mm kcl = 74.55 g/mol mm kclo3 = 122.55 g/mol if 4.00 moles of kclo3 are totally consumed, how many grams of oxygen gas would be produced? 192 g 6.00 g 85.3 g 735 g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, KayPink5723
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3



Do you know the correct answer?
The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info:...
Questions in other subjects:

Spanish, 04.03.2021 18:00





History, 04.03.2021 18:00

Mathematics, 04.03.2021 18:00


English, 04.03.2021 18:00