
Chemistry, 17.09.2019 19:00, chrisroman152
In the chemical reaction below, 300 grams of reactant a reacted completely with 100 grams of reactant b, to form the product ab. how many grams of the product, ab, would be formed in this complete reaction?
slightly less than 400 grams, because some atoms always disappear into thin air when a chemical reaction occurs.
400 grams of ab
200 grams of ab
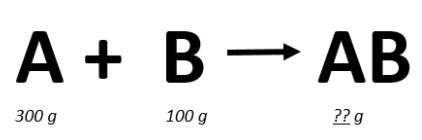

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1

Chemistry, 23.06.2019 10:30, jetblackcap
An atom that gains or loses one or more electrons is called a(n)
Answers: 1
Do you know the correct answer?
In the chemical reaction below, 300 grams of reactant a reacted completely with 100 grams of reactan...
Questions in other subjects:



Social Studies, 02.03.2021 02:30

Biology, 02.03.2021 02:30

Mathematics, 02.03.2021 02:30


Mathematics, 02.03.2021 02:30



English, 02.03.2021 02:30






