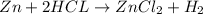When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipitate, zinc chloride (zncl2) was produced. she found that the mass of the zinc chloride was 93 g. since the law of conservation of matter stated that matter can't be created or destroyed, she was puzzled about the difference in mass. what is the best explanation for the missing 2 g?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, juansantos7b
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
Do you know the correct answer?
When kay added 30 g of hydrochloric acid (hcl) to 65 g of zn, bubbles appeared and a white precipita...
Questions in other subjects:



Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01



Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01

History, 12.08.2020 08:01