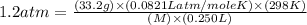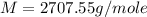Chemistry, 27.07.2019 18:40, krazziekidd2p845ri
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 33.2 g of the compound in sufficient water to form 250 ml of solution. the solution has an osmotic pressure of 1.2 atm at 25 °c. what is the molar mass (g/mole) of the compound?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 08:30, lpssprinklezlps
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3
Do you know the correct answer?
An aqueous solution of a soluble compound (a nonelectrolyte) is prepared by dissolving 33.2 g of the...
Questions in other subjects:

Mathematics, 20.10.2019 11:10




Social Studies, 20.10.2019 11:10



Computers and Technology, 20.10.2019 11:10



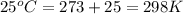
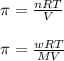
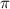 = osmotic pressure
= osmotic pressure