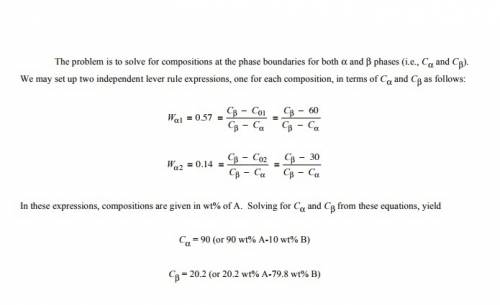
For alloys of two hypothetical metals a and b, there exist an α, a-rich phase and a β, b-rich phase. from the mass fractions of both phases for two different alloys (given below), which are at the same temperature, determine the composition of the phase boundary (or solubility limit) for (a)α and (b)β phases at this temperature.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2


Chemistry, 23.06.2019 07:10, skatingflower
What additive are in a lavender tube for phlebotomists
Answers: 1
Do you know the correct answer?
For alloys of two hypothetical metals a and b, there exist an α, a-rich phase and a β, b-rich phase....
Questions in other subjects:

Mathematics, 04.07.2019 17:10


Mathematics, 04.07.2019 17:10

Business, 04.07.2019 17:10


History, 04.07.2019 17:10


History, 04.07.2019 17:10