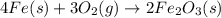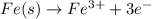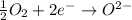Chemistry, 27.07.2019 11:10, graymonky12
Why is rusted iron an example of an oxidation–reduction reaction? electrons are exchanged?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, sammysosa121832
The ph of carrots are 5.0 how it is classified a. acidic b. basic c. indicator d. neutral
Answers: 2

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Do you know the correct answer?
Why is rusted iron an example of an oxidation–reduction reaction? electrons are exchanged?...
Questions in other subjects:

Biology, 24.10.2019 08:43







Mathematics, 24.10.2019 08:43