A- gases do not have mass.
b- gases are less dense than liquids.
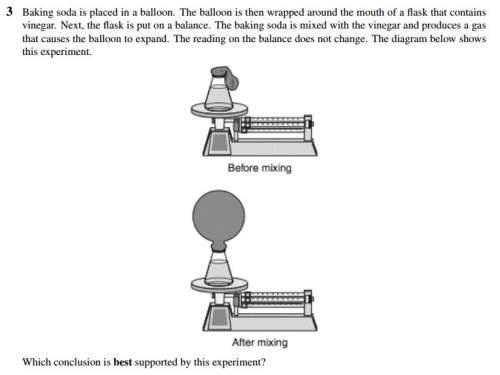
c- there is no change i...

Chemistry, 10.11.2019 23:31, pablogonzaleztellez
A- gases do not have mass.
b- gases are less dense than liquids.
c- there is no change in mass during a chemical reaction.
d-the reactants do not change during a chemical reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 07:00, jboii11
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample? a. naphthalene, a molecular solid with the formula c10h8 b. silica, a network solid held together by covalent bonds with the formula sio2 c. calcium chloride, an ionic compound with the formula cacl2 d. water, an molecular compound with the formula h2o
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Chemistry, 14.06.2021 16:30

English, 14.06.2021 16:30

History, 14.06.2021 16:30

Advanced Placement (AP), 14.06.2021 16:30


Mathematics, 14.06.2021 16:30

English, 14.06.2021 16:30


Chemistry, 14.06.2021 16:30






