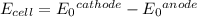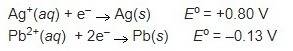An electrochemical cell is powered by the half reactions shown below.
...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3

Chemistry, 23.06.2019 10:30, villarrealc1987
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 11:50, halllawson
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 30.06.2019 12:10

English, 30.06.2019 12:10

Mathematics, 30.06.2019 12:10






Social Studies, 30.06.2019 12:20