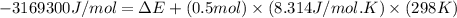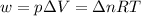Chemistry, 26.07.2019 04:00, tonytashaqua
Calculate the work (w) and δeo, in kj, at 298 k and 1 atm pressure, for the combustion of one mole of c6h6 (g). first write and balance the equation. the products will be co2 (g) and h2o (g). the value of δho for this reaction is -3169.3 kj/mol.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1


Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Do you know the correct answer?
Calculate the work (w) and δeo, in kj, at 298 k and 1 atm pressure, for the combustion of one mole o...
Questions in other subjects:

Law, 27.02.2021 07:10


Mathematics, 27.02.2021 07:10


Mathematics, 27.02.2021 07:10

Computers and Technology, 27.02.2021 07:10

Mathematics, 27.02.2021 07:10




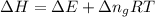
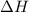 = change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol
= change in enthalpy = -3169.3 kJ/mol = -3169300 J/mol = change in internal energy
= change in internal energy = change in moles
= change in moles