
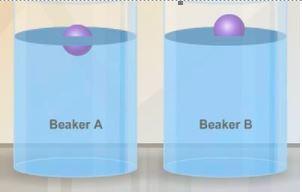
Identical objects are placed in beaker a and beaker b. the objects float as shown in the diagram. what can you conclude about the liquid in each of the beakers? a question 6 options: the liquid in beaker b has a greater density than the liquid in beaker a. the liquid in beaker a has a greater density than the liquid in beaker b. the liquid in beaker a has a greater density than the objects. the liquid in beaker b is less dense than the objects. both liquids are less dense than the objects. i think the answers are b or d me!

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
Do you know the correct answer?
Identical objects are placed in beaker a and beaker b. the objects float as shown in the diagram. wh...
Questions in other subjects:

Mathematics, 28.05.2020 06:01

Mathematics, 28.05.2020 06:01

Mathematics, 28.05.2020 06:01


English, 28.05.2020 06:01


Chemistry, 28.05.2020 06:01



Mathematics, 28.05.2020 06:01






