
Chemistry, 23.07.2019 22:40, anthonyalvar6636
In each pair, identify the solution that will have a higher boiling point. explain. 1. 1.50 moles of lioh (strong electrolyte) and 3.00 moles of koh (strong electrolyte) each in 1.0 kg of water 2. 0.40 mole of al(no3)3 (strong electrolyte) and 0.40 mole of cscl (strong electrolyte) each in 1.0 kg of water

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Do you know the correct answer?
In each pair, identify the solution that will have a higher boiling point. explain. 1. 1.50 moles of...
Questions in other subjects:


Geography, 13.01.2021 20:10



Mathematics, 13.01.2021 20:10


Chemistry, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

Mathematics, 13.01.2021 20:10

History, 13.01.2021 20:10


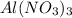 in 1.0 kg of water.
in 1.0 kg of water.
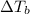 is the elevation in boiling point, i is the Van't hoff factor, m is the molality of the solution and
is the elevation in boiling point, i is the Van't hoff factor, m is the molality of the solution and 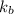 is the molal elevation constant for the solvent used.
is the molal elevation constant for the solvent used.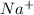 and
and 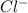 . So, the value of i for NaCl is 2.
. So, the value of i for NaCl is 2. 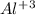 ion and three
ion and three 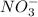 ions. So, the value of i for this is 4.
ions. So, the value of i for this is 4. 



