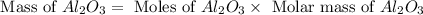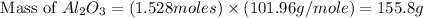Chemistry, 23.07.2019 13:20, paranoidbih
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4al(s) + 3o2(g) ® 2al2o3(s) [balanced] a mixture of 82.49 g of aluminum ( = 26.98 g/mol) and 117.65 g of oxygen ( = 32.00 g/mol) is allowed to react. what mass of aluminum oxide ( = 101.96 g/mol) can be formed?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 23:00, liv467
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
Do you know the correct answer?
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts...
Questions in other subjects:

History, 20.05.2021 01:10



Mathematics, 20.05.2021 01:10

Social Studies, 20.05.2021 01:10

Mathematics, 20.05.2021 01:10


Mathematics, 20.05.2021 01:10



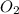 = 117.65 g
= 117.65 g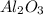 = 101.96 g/mole
= 101.96 g/mole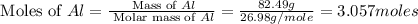
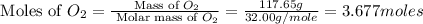
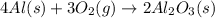
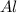 react with 3 mole of
react with 3 mole of 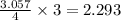 moles of
moles of 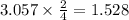 moles of
moles of