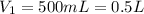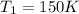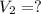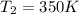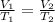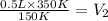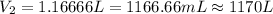Chemistry, 23.07.2019 10:00, ciarrap552
The temperature of a 500. ml sample of gas increases from 150. k to 350. k. what is the final volume of the sample of gas, if the pressure and moles in the container is kept constant? 0.0095 ml 110. ml 0.0470 ml 210. ml 1170 ml

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
Do you know the correct answer?
The temperature of a 500. ml sample of gas increases from 150. k to 350. k. what is the final volume...
Questions in other subjects:

Health, 20.09.2019 10:50

Biology, 20.09.2019 10:50

Physics, 20.09.2019 10:50

History, 20.09.2019 10:50





Mathematics, 20.09.2019 10:50