
Chemistry, 22.07.2019 18:50, haileysmile2006
The specific heat of copper is 0.385 j/(g · °c). if 34.2 g of copper, initially at 24.0°c, absorbs 4.689 kj, what will be the final temperature of the copper?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30, quantamagic
Word equation for k(s)+h2o(l) yield koh(aq) + h2(g)
Answers: 1
Do you know the correct answer?
The specific heat of copper is 0.385 j/(g · °c). if 34.2 g of copper, initially at 24.0°c, absorbs 4...
Questions in other subjects:


History, 12.11.2020 04:50

English, 12.11.2020 04:50



Biology, 12.11.2020 04:50


History, 12.11.2020 04:50



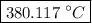
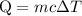 …… (1)
…… (1) is the change in temperature of copper.
is the change in temperature of copper.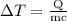 …… (2)
…… (2)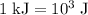
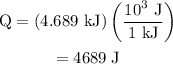
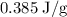 .
.
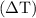 can be calculated as follows:
can be calculated as follows: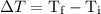 …… (3)
…… (3)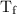 is the final temperature.
is the final temperature.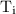 is the initial temperature.
is the initial temperature.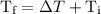 …… (4)
…… (4)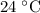 .
.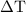 is
is 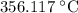
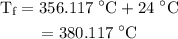
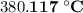 .
.



