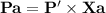Chemistry, 22.07.2019 11:00, mercedesamatap21hx0
Three gases (8.00 g of methane, ch4, 18.0 g of ethane, c2h6, and an unknown amount of propane, c3h8) were added to the same 10.0-l container. at 23.0 ∘c, the total pressure in the container is 5.50 atm . calculate the partial pressure of each gas in the container.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
Do you know the correct answer?
Three gases (8.00 g of methane, ch4, 18.0 g of ethane, c2h6, and an unknown amount of propane, c3h8)...
Questions in other subjects: