
Chemistry, 20.07.2019 15:10, Benysod402
The main assumptions of the kinetic molecular theory of gases are: 1. gases are made up of molecules which are relatively far apart. 2. the molecules are in motion at high speeds. 3. the molecules are perfectly elastic. 4. increase in temperature increases the kinetic energy of the molecules. the assumption that explains charles' law is: 1 2 3 4 1 and 3

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1
Do you know the correct answer?
The main assumptions of the kinetic molecular theory of gases are: 1. gases are made up of molecule...
Questions in other subjects:



Biology, 13.10.2019 04:30

Social Studies, 13.10.2019 04:30







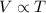 (at constant pressure)
(at constant pressure)



