
Aqueous hydrobromic acid hbr will react with solid sodium hydroxide naoh to produce aqueous sodium bromide nabr and liquid water h2o . suppose 20.2 g of hydrobromic acid is mixed with 5.7 g of sodium hydroxide. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 2 significant digits.

Answers: 1
Similar questions

Chemistry, 06.10.2019 18:30, alinamartinez9p752cj
Answers: 3

Chemistry, 02.11.2019 05:31, davidoj13
Answers: 1
Do you know the correct answer?
Aqueous hydrobromic acid hbr will react with solid sodium hydroxide naoh to produce aqueous sodium b...
Questions in other subjects:


Physics, 21.07.2019 15:30

Physics, 21.07.2019 15:30


History, 21.07.2019 15:30


Social Studies, 21.07.2019 15:30

Chemistry, 21.07.2019 15:30



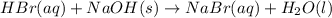
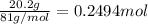
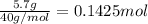
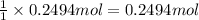 of NaOH.
of NaOH.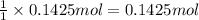 of HBr.
of HBr.



