Chemistry, 20.07.2019 05:40, nanamath5662
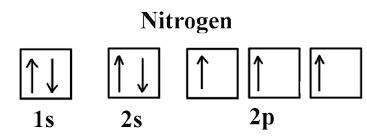
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic represents the correct configuration for a nitrogen atom in its ground state. which one is the correct electron configuration?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1


Do you know the correct answer?
Four possible electron configurations for a nitrogen atom are shown below, but only one schematic re...
Questions in other subjects:


Mathematics, 08.07.2019 03:30



History, 08.07.2019 03:30

Social Studies, 08.07.2019 03:30



Mathematics, 08.07.2019 03:30

Mathematics, 08.07.2019 03:30