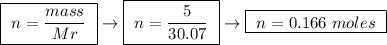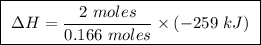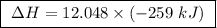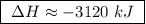Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, lilyclairehutson
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1


Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2
Do you know the correct answer?
At constant pressure, the combustion of 5.00 g of c2h6(g) releases 259 kj of heat. what is δh for th...
Questions in other subjects:


Biology, 20.11.2020 14:40

Mathematics, 20.11.2020 14:40

Biology, 20.11.2020 14:40


Arts, 20.11.2020 14:40



English, 20.11.2020 14:40

Chemistry, 20.11.2020 14:40


 .
.