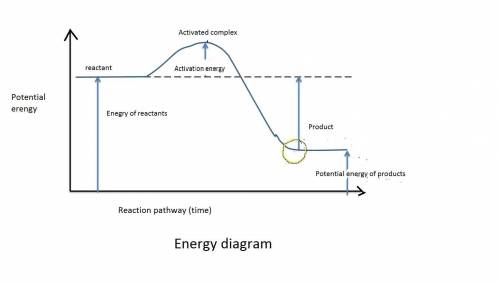
Chemistry, 17.07.2019 03:40, ryanbransky
Reactions cannot occur without a certain minimum amount of energy. what is this minimum amount of energy called? a. the activation energy b. the activated complex c. the kinetic energy of reactants d. the potential energy of products

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, nothingworksoutforme
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 13:10, kellinvagneur
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
Do you know the correct answer?
Reactions cannot occur without a certain minimum amount of energy. what is this minimum amount of en...
Questions in other subjects: