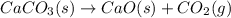Chemistry, 16.07.2019 19:00, DivineMemes420
Which statement below is not true of decomposition reactions? they usually require the addition of energy for the reaction to occur. they produce more than one product at the end of the reaction. they begin with more active reactants and end with less active products. they have one compound as the reactant at the beginning of the reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, gilbert325
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1
Do you know the correct answer?
Which statement below is not true of decomposition reactions? they usually require the addition of...
Questions in other subjects:


History, 18.02.2021 18:00

History, 18.02.2021 18:00

Mathematics, 18.02.2021 18:00

Social Studies, 18.02.2021 18:00

English, 18.02.2021 18:00



English, 18.02.2021 18:00

Mathematics, 18.02.2021 18:00