

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
A0.8838-g sample of an ionic compound containing bromide ions and an unknown metal cation is dissolv...
Questions in other subjects:



Biology, 15.10.2019 02:00

Mathematics, 15.10.2019 02:00





History, 15.10.2019 02:00

History, 15.10.2019 02:00


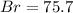 %
%
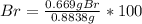 %
%



