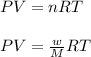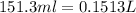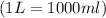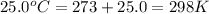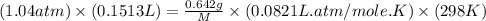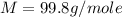Chemistry, 13.07.2019 13:30, lizdominguez101
A0.642g sample of an unknown gas was collected over water at 25.0°c and 1.04 atm. the collection cylinder contained 151.3 ml of gas after the sample was released. find the molar mass of the unknown gas.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 12:30, hala201490
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Do you know the correct answer?
A0.642g sample of an unknown gas was collected over water at 25.0°c and 1.04 atm. the collection cyl...
Questions in other subjects:



World Languages, 07.09.2020 08:01

Chemistry, 07.09.2020 08:01



Computers and Technology, 07.09.2020 08:01