
Chemistry, 13.07.2019 13:20, wendyyy1214
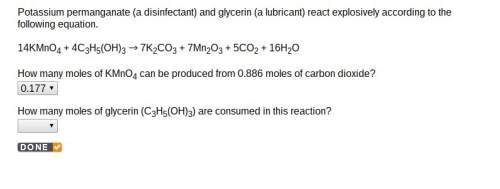
How many moles of glycerin (c3h5(oh)3) are consumed in this reaction? 14kmno4 + 4c3h5(oh)3 es001-1.jpg 7k2co3 + 7mn2o3 + 5co2 + 16h2o

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, marcusajns
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Do you know the correct answer?
How many moles of glycerin (c3h5(oh)3) are consumed in this reaction? 14kmno4 + 4c3h5(oh)3 es001-1....
Questions in other subjects:

Mathematics, 01.12.2020 22:00


Mathematics, 01.12.2020 22:00

Mathematics, 01.12.2020 22:00


History, 01.12.2020 22:00

Mathematics, 01.12.2020 22:00








