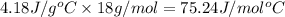Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1


Do you know the correct answer?
The specific heat of water is 4.18 j/(g⋅∘ c. calculate the molar heat capacity of water....
Questions in other subjects:




Business, 06.11.2020 17:20


English, 06.11.2020 17:20




Computers and Technology, 06.11.2020 17:20