
Chemistry, 16.09.2019 13:30, Uhmjujiooo4220
Enough of a monoprotic acid is dissolved in water to produce a 0.0116 m solution. the ph of the resulting solution is 2.35. calculate the ka for the acid.

Answers: 1
Similar questions


Do you know the correct answer?
Enough of a monoprotic acid is dissolved in water to produce a 0.0116 m solution. the ph of the resu...
Questions in other subjects:




Mathematics, 22.03.2021 01:00

World Languages, 22.03.2021 01:00

Health, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00


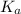 = 1.72 × 10⁻³
= 1.72 × 10⁻³![K_{a} = \frac{[H_{3} O^{+}][ A^{-}] }{[HA]}](/tpl/images/0233/1446/cf7d2.png)
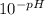 mol/L=
mol/L= 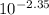 mol/L = 4.47 × 10⁻³ mol/L
mol/L = 4.47 × 10⁻³ mol/L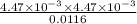 = 1.72 × 10⁻³
= 1.72 × 10⁻³



