
Chemistry, 20.08.2019 03:00, dheydar9377
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final reading is 38.55 ml. using the correct number of significant figures, determine the density of the metal = g/ml first determine volume of metal: report result to the same number of decimal places as the number with the least number of decimal places. use this volume in calculation. second determine density of metal: d=m/v report result to the same number of significant figures as the number with the least number of significant figures. do not use exponential notation in answer.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 19:10, krisandlance
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
Do you know the correct answer?
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final rea...
Questions in other subjects:


History, 06.10.2019 19:30

Health, 06.10.2019 19:30



Social Studies, 06.10.2019 19:30

Mathematics, 06.10.2019 19:30

Mathematics, 06.10.2019 19:30


Physics, 06.10.2019 19:30


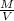 =
= 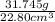 = 1.392 g/cm³
= 1.392 g/cm³



