The empirical formula of the hydrocarbon is 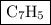 .
.
Further explanation:
Empirical formula:
It is atom’s simplest positive integer ratio in the compound. It may or may not be same as that of molecular formula. For example, empirical formula of sulfur dioxide is SO.
Combustion reactions:
These are the reactions that take place when hydrocarbons are burnt in the presence of oxygen to form carbon dioxide and water. These are also referred to as burning.
Example of combustion reactions are as follows:
(a) 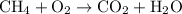
(b) 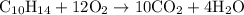
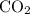 is formed as a product during combustion reactions.
is formed as a product during combustion reactions.
Step 1: 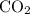 is formed as a product during combustion reactions. Initially, we have to calculate the moles of
is formed as a product during combustion reactions. Initially, we have to calculate the moles of 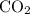 . The formula to calculate moles of
. The formula to calculate moles of 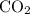 is as follows:
is as follows:
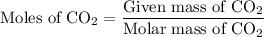 ...... (1)
...... (1)
The given mass of 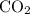 is 86.5 g.
is 86.5 g.
The molar mass of 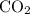 is 44 g/mol.
is 44 g/mol.
Substitute these values in equation (1).
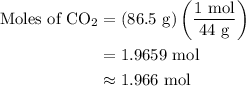
Step 2: During combustion, one mole of carbon reacts to form one mole of 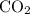 .So the mass of C in the hydrocarbon is calculated as follows:
.So the mass of C in the hydrocarbon is calculated as follows:
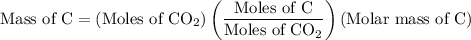 ...... (2)
...... (2)
The moles of 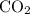 is 1.966 mol.
is 1.966 mol.
The molar mass of C is 12 g/mol.
The mole of C is 1 mol.
The moles of 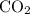 is 1 mol.
is 1 mol.
Substitute these values in equation (2).
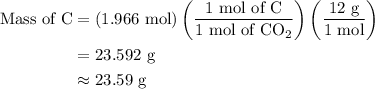
Step 3: Since the hydrocarbon consists of only carbon and hydrogen. The mass of hydrogen is calculated as follows:
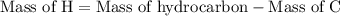 ...... (3)
...... (3)
The mass of hydrocarbon is 25 g.
The mass of carbon is 23.59 g.
Substitute these values in equation (3).
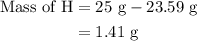
The formula to calculate moles of H is as follows: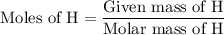 ...... (4)
...... (4)
The given mass of H is 1.41 g.
The molar mass of H is 1.01 g/mol.
Substitute these values in equation (4).
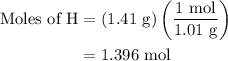
The moles of carbon and hydrogen present in hydrocarbon are to be written with their corresponding subscripts. So the preliminary formula becomes,
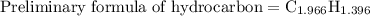
Step 4: Each of the subscripts is divided by the smallest subscript to get the empirical formula. In this case, the smallest one is 1.39. So the empirical formula of hydrocarbon is written as follows:

Step 5: Multiply each subscript of the empirical formula by 5, we get the final empirical formula as follows:
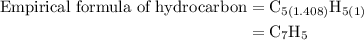
Therefore, the empirical formula of hydrocarbon is 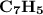 .
.
Learn more:
1. Calculate the moles of ions in the solution:
2. Calculate the moles of chlorine in 8 moles of carbon tetrachloride:
Answer details:
Grade: Senior School
Subject: Chemistry
Chapter: Stoichiometry of formulas and equations
Keywords: empirical formula, C, H, C7H5, moles of CO2, C, H, 5, preliminary formula, whole number.
















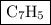 .
.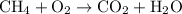
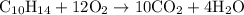
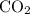 is formed as a product during combustion reactions.
is formed as a product during combustion reactions.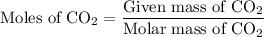 ...... (1)
...... (1)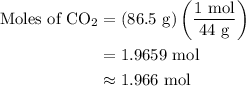
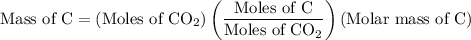 ...... (2)
...... (2)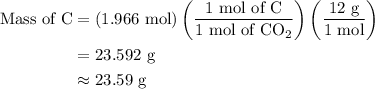
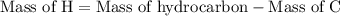 ...... (3)
...... (3)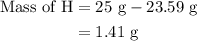
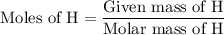 ...... (4)
...... (4)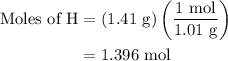
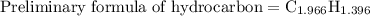

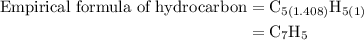
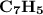 .
.




