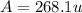Chemistry, 08.07.2019 23:10, KillerSteamcar
Anewly discovered element, y, has two naturally occurring isotopes. 90.3 percent of the sample is an isotope with a mass of 267.8 u, and 9.7 percent of the sample is an isotope with a mass of 270.9 u. what is the weighted average atomic mass for this element? 269.4 u 268.1 u 269.0 u 270.6 u

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 23.06.2019 00:00, sanaiajohnson56
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Do you know the correct answer?
Anewly discovered element, y, has two naturally occurring isotopes. 90.3 percent of the sample is an...
Questions in other subjects:

Mathematics, 21.02.2021 02:10


Engineering, 21.02.2021 02:10

Mathematics, 21.02.2021 02:10


History, 21.02.2021 02:10

Mathematics, 21.02.2021 02:10

Spanish, 21.02.2021 02:10

Mathematics, 21.02.2021 02:10

Chemistry, 21.02.2021 02:10


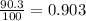
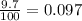
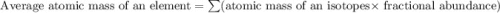
![A=\sum[267.8\times 0.903+270.9\times 0.097]](/tpl/images/0067/2857/bf750.png)