
Chemistry, 07.07.2019 02:20, allhailkingmilkdud
The balanced equation for combustion in an acetylene torch is shown below: 2c2h2 + 5o2 → 4co2 + 2h2o the acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2. how many moles of co2 are produced when 35.0 mol c2h2 react completely?

Answers: 2
Similar questions


Chemistry, 16.07.2019 08:00, VamLS7472
Answers: 1


Chemistry, 09.10.2019 23:30, tre3731
Answers: 1
Do you know the correct answer?
The balanced equation for combustion in an acetylene torch is shown below: 2c2h2 + 5o2 → 4co2 + 2h2...
Questions in other subjects:

Mathematics, 20.03.2021 14:00

Mathematics, 20.03.2021 14:00


Mathematics, 20.03.2021 14:00

English, 20.03.2021 14:00

Mathematics, 20.03.2021 14:00


Mathematics, 20.03.2021 14:00



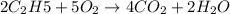
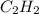 combine with 5 moles of oxygen
combine with 5 moles of oxygen 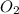 to produce 4 moles of carbon dioxide
to produce 4 moles of carbon dioxide 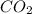 .
.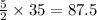 moles of oxygen
moles of oxygen 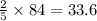 moles of acetylene
moles of acetylene 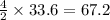 moles of of carbon dioxide
moles of of carbon dioxide 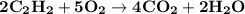
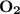 , react with
, react with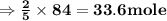 of acetone
of acetone 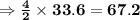 of Carbon dioxide
of Carbon dioxide 




