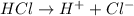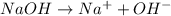1what do strong acids and strong bases have in common? a they both partially dissociate, with reverse reactions occurring. b they both dissociate completely, with little or no reverse reactions. c they both remain intact when placed in water, with no dissociation taking place. d they both dissociate completely, with reverse reactions constantly taking place.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, jescanarias22
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Do you know the correct answer?
1what do strong acids and strong bases have in common? a they both partially dissociate, with reve...
Questions in other subjects:




World Languages, 06.07.2021 03:30

English, 06.07.2021 03:30

Mathematics, 06.07.2021 03:30


World Languages, 06.07.2021 03:30